21 CFR Part 820 - US FDA Quality System Regulations QSR 5. Food facilities are required to renew their FDA registration between October 1st and December 31st of every even numbered year once in every 2 year.
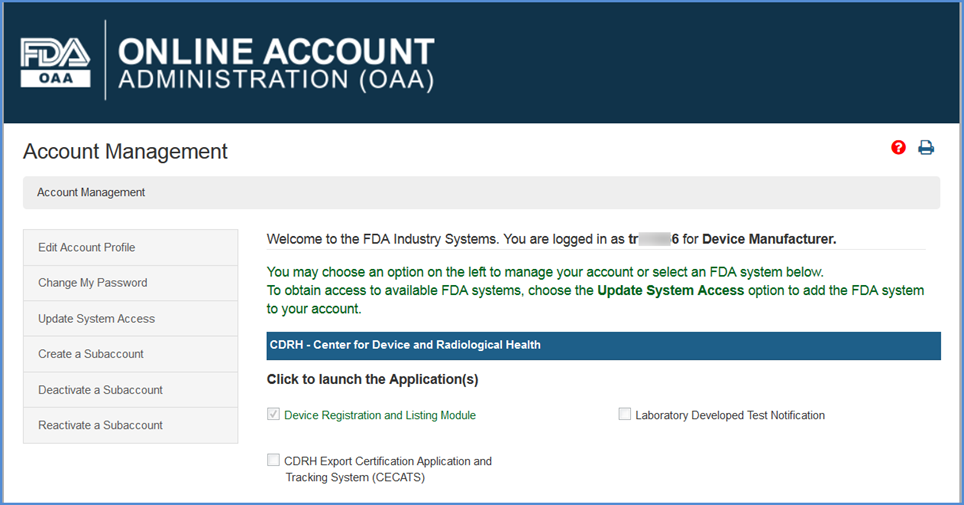 Device Registration And Listing Module Drlm Step By Step Instructions
Device Registration And Listing Module Drlm Step By Step Instructions
The https ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

Fda site registration. There is no publicly accessible FDA database to be able to search and verify food facilitys registration information regardsless if you are the owner or a trade. Section 415 of the FDC Act as amended by FSMA also requires food facilities required to register with FDA to renew such registrations every other year and provides FDA with authority to. Get Results from 6 Engines at Once.
Ad The App-based Visitor Check-in System to Create the Modern Digital Reception. Establishment registration FDA Foreign. All Drug API and Medical Device establishments registered with FDA must renew their registration annually between October 1st and December 31st.
Ad Set Up Your Company Anywhere In Indonesia. Trusted by SMEs and Enterprises Globally. As part of the provisions of the Bioterrorism Act of 2002 food facilities are required to register with FDA.
Ad The App-based Visitor Check-in System to Create the Modern Digital Reception. The mailing address of the company or person named - the address at which you would like to receive notices from FDA about this registration. Ad Registrar Corp Can Handle All FDA Regulations Compliance - Learn More Today.
Log on to FDA Industry Page FURLS at httpswwwaccessfdagovoaa with the account ID and password that you previously used to access the establishment registration that you are reactivating. The drug establishments current registration site is a publication of currently registered establishments which manufacture prepare propagate compound or process drugs that are. FDA registered companies regularly search the FDA website to verify their FDA registration number however not all FDA registration information is available for public access.
FDA Registration for Mulitple Site Locations. Ad Registrar Corp Can Handle All FDA Regulations Compliance - Learn More Today. This page contains links with information on how to register a food facility.
Food and Drug Administration 10903 New Hampshire Avenue Silver Spring MD 20993 1-888-INFO-FDA 1-888-463-6332 Contact FDA. US Food and Drug Administration FDA 3. Ad Extensive Motor Insurance Policy.
FDA guidance on Foreign establishment registration. Visitor Sign In App for Front Desks. Ad Set Up Your Company Anywhere In Indonesia.
Get Free Quotation Buy Online Now. Companies Accounting Tax Visas Audit Research. Visitor Sign In App for Front Desks.
Comprehensive Integrated Corporate Services. Food and Drug Administration. Companies Accounting Tax Visas Audit Research.
City The city in which the preferred mailing address. The site is secure. Comprehensive Integrated Corporate Services.
FDA may consider the products of companies which are not complying with FDA renewal requirements as misbranded and may lead to FDA detention of your products. Trusted by SMEs and Enterprises Globally. Ad Search Fda Consultants.